CAPSAICINOIDS:
The chemical components of a hot pepper that causes the familiar burning sensation, are all members of a family of compounds known as capsaicinoids.
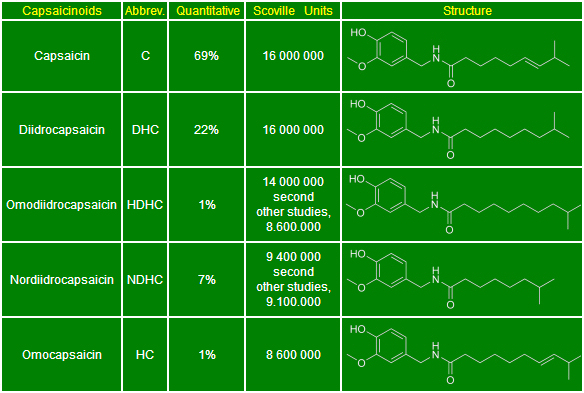
The natural capsaicinoids are: Capsaicin (C), Dihydrocapsaicin (DHC), Homodihydrocapsaicin (HDHC), Nordihydrocapsaicin (NDHC), Homocapsaicin (HC). The concentration of capsaicinoids in peppers varies from species to species, from variety to variety and stimulate and act differently. In a study conducted by Anna Krajewska and John Powers in 1988, noted that the capsaicinoids, in very dilute solutions, produce different kinds of hotness. Capsaicin and Dihydrocapsaicin act almost the same way. Their action is concentrated to half mouth and half of the palate and the throat and the back of the tongue, developing rapidly with a persistent effect. The Homodihydrocapsaicin has a very strong and irritating, "paralyzing", which is concentrated in the throat at the back of the tongue and palate. It develops slowly and is difficult to remove. The Nordihydrocapsaicin, in contrast, produces a less irritating sensation that focuses in front of the mouth and palate, has a fruity flavor described as sweet and spicy. Develops immediately, but fades quickly. Therefore, depending on the percentage of specific capsaicinoids present in the sample chili, is rather straightforward to understand how some types of peppers are or appear to be more hot than others, even if the same value in the Scoville scale. Some varieties burn mainly on the tongue, while others burn in the back of the throat. Thus by varying the distribution of capsaicinoids, there are several types of hotness. Most of the hotness, 80 to 90% is composed of: Capsaicin and Dihydrocapsaicin. The rest of the contents capsaicinoids is constituted by one or more minor capsaicinoids. Numerous studies have demonstrated that the two major capsaicinoids are also the two capsaicinoids hottest. The capsaicinoids alkaloids were recognized as distinct from the Japanese S. Kosuge and Y. Inagaki in 1964. If there are eleven in nature, and various plastics, such as the n-nonanoic acid vanillilamide (VNA).